ABSTRACT
Introduction
Point-of-care molecular diagnostics offer solutions to the limited diagnostic availability and accessibility in resource-limited settings.
During the COVID-19 pandemic, molecular diagnostics became essential tools for accurately detecting and monitoring SARS-CoV-2. The unprecedented demand for molecular diagnostics presented challenges and catalyzed innovations which may provide lessons for the future selection of point-of-care molecular diagnostics.
Areas Covered
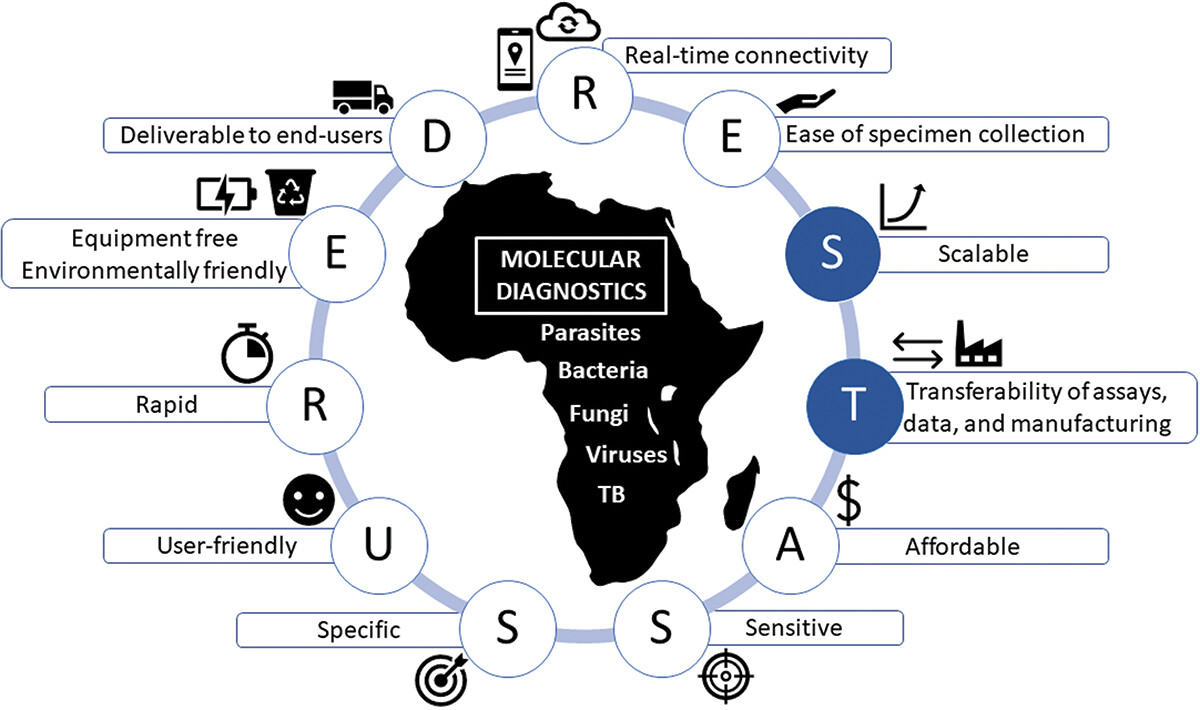
We searched PubMed from January 2020-August 2023 to identify lessons learned from the COVID-19 pandemic which may impact the selection of point-of-care molecular diagnostics for future use in sub-Saharan Africa. We evaluated this in the context of REASSURED criteria (Real-time connectivity; Ease of specimen collection; Affordable; Sensitive; Specific; User-friendly; Rapid and robust; Equipment free; and Deliverable to users at the point of need) for point-of-care diagnostics for resource-limited settings.
Expert Opinion
The diagnostic challenges and successes during the COVID-19 pandemic affirmed the importance of the REASSURED criteria but demonstrated that these are not sufficient to ensure new diagnostics will be appropriate for public health emergencies. Capacity for rapid scale-up of diagnostic testing and transferability of assays, data, and technology are also important, resulting in updated REST-ASSURED criteria. Few diagnostics will meet all criteria, and trade-offs between criteria will need to be context specific.
Read the Full Article here